OGSIVEO was studied in the largest completed* Phase 3 trial in adult patients with desmoid tumors
DeFi: An international, multicenter, randomized (1:1), double-blind, placebo-controlled, Phase 3 study of OGSIVEO in patients with progressing desmoid tumors not amenable to surgery. Patients had treatment-naïve, refractory, or recurrent disease (N=142).1,2
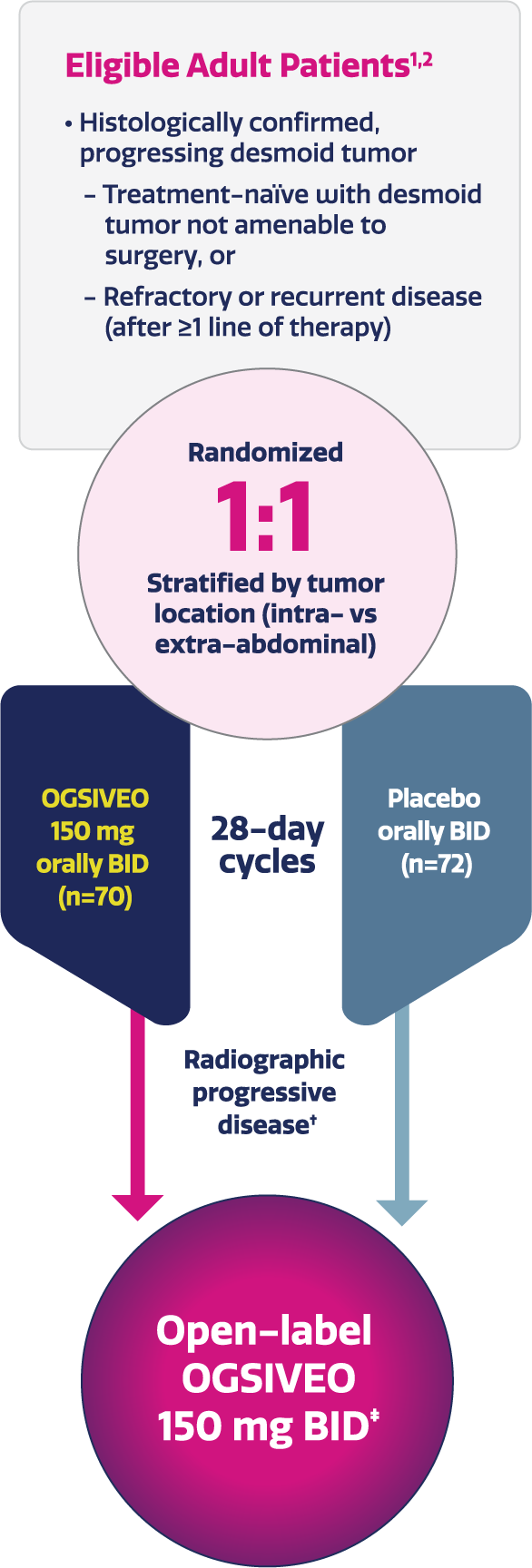
- All patients had histologically confirmed desmoid tumors that had progressed ≥20% by RECIST v1.1 within 12 months before screening2
- If patients had multiple target tumors that were located in the intra- and extra-abdominal locations, they were classified as intra-abdominal2
- Patients were randomized to receive 150 mg OGSIVEO or placebo orally twice daily until disease progression or unacceptable toxicity1
- Tumor imaging occurred every 3 months1
Completed double-blind, randomized, Phase 3 trial in adult patients with desmoid tumors.1,2
Imaging-based progression or completion of the primary analysis.2
Eligible patients were given the option to enroll in the open-label extension phase.2
BID, twice daily; DeFi, Desmoid Fibromatosis; RECIST, Response Evaluation Criteria in Solid Tumors.
Key End Points in the DeFi Study
Primary End Point
Progression-Free Survival1,2
- Progression-free survival was defined as the time from randomization until the date of imaging-based or clinical progression or death2
- Progression-free survival was based on RECIST v1.1 as assessed by blinded independent central review or on clinical progression by the investigator (and confirmed by independent review)1
- Clinical progression required worsening of symptoms resulting in a global deterioration of health status causing the permanent discontinuation from trial treatment and the initiation of emergent treatment (e.g., radiotherapy, surgery, or systemic therapy including chemotherapy or tyrosine kinase inhibitors) for desmoid tumors1
Key Secondary Efficacy End Points§
Objective Response Rate1,2
- Objective response rate was defined as complete response or partial response according to RECIST v1.1. Assessed by blinded independent central review1,2,‖
- Partial response was defined as a ≥30% decrease in the sum of the longest diameters of target tumors2
- Complete response was defined as disappearance of all target and non-target tumors2
Worst Pain Intensity (change from baseline at Cycle 10¶)1,2
- Patient-reported worst pain intensity was assessed daily using item 3 of the Brief Pain Inventory-Short Form (BPI-SF), an 11-point numerical rating scale ranging from 0 (“no pain”) to 10 (“pain as bad as you can imagine”) and averaged over 7 days prior to each visit1,2
Additional secondary efficacy end points were evaluated in the DeFi study.2
Confirmed by repeat assessments that were performed no less than 4 weeks after the criteria for response were first met.2
Each cycle was 28 days.2
FAP, familial adenomatous polyposis; RECIST, Response Evaluation Criteria in Solid Tumors.
Baseline Characteristics1,2
The DeFi study included a broad range of patients who were representative of a real-world population with desmoid tumors1,2
- All patients experienced tumor progression ≥20% as measured by RECIST v1.1 within 12 months prior to treatment initiation1,2
- Patient-reported pain at baseline in the DeFi study:
- 50% of patients had a BPI-SF item 3 (worst pain intensity) score of ≥21
- 41% of patients had uncontrolled pain (BPI-SF worst pain intensity score >4)2
Baseline demographics and disease-related characteristics1,2
Baseline demographics and disease-related characteristics1,2
| OGSIVEO (n=70) | Placebo (n=72) | |
|---|---|---|
| Age, median (min, max) | 33.5 years (18, 73) | 34.5 years (18, 76) |
| Sex, n (%) | ||
| Male | 25 (36%) | 25 (35%) |
| Female | 45 (64%) | 47 (65%) |
| Females of reproductive potential* | 37 (53%) | 37 (51%) |
| Race, n (%) | ||
| White | 64 (91%) | 54 (75%) |
| Black or African American | 4 (6%) | 5 (7%) |
| Asian | 1 (1%) | 3 (4%) |
| Other | 1 (1%) | 10 (14%) |
| Family history of FAP, n (%) | 11 (16%) | 13 (18%) |
| Somatic mutations, n (%) | 52 (74%) | 53 (74%) |
| APC | 11 (16%) | 11 (15%) |
| CTNNB1 | 43 (61%) | 42 (58%) |
| No identified mutation | 0 | 1 (1%) |
| Not analyzed | 18 (26%) | 18 (25%) |
| Target tumor location, n (%) | ||
| Intra-abdominal | 17 (24%) | 18 (25%) |
| Extra-abdominal | 53 (76%) | 54 (75%) |
| Multifocal disease, n (%) | 27 (39%) | 31 (43%) |
| Desmoid tumor treatment status, n (%) | ||
| Treatment-naïve | 18 (26%) | 14 (19%) |
| Refractory/recurrent following prior treatment | 52 (74%) | 58 (81%) |
| Prior therapies, n (%) | ||
| Surgery | 31 (44%) | 44 (61%) |
| Radiation | 16 (23%) | 16 (22%) |
| Systemic therapy | 43 (61%) | 44 (61%) |
| Chemotherapy | 24 (34%) | 27 (38%) |
| Tyrosine kinase inhibitor | 23 (33%) | 24 (33%) |
Defined in the trial protocol as females between menarche and confirmed menopause (ie, 12 months since last menstruation) with intact ovarian function. Based on the investigator’s judgment.2
APC, adenomatous polyposis coli; BPI-SF, Brief Pain Inventory-Short Form; CTNNB1, catenin beta 1; DeFi, Desmoid Fibromatosis; FAP, familial adenomatous polyposis; RECIST, Response Evaluation Criteria in Solid Tumors.
OGSIVEO demonstrated powerful efficacy in the DeFi study1
OGSIVEO significantly improved progression-free survival1
Primary End Point:Patients receiving OGSIVEO achieved a 71% reduction in the risk of disease progression or death vs placebo (HR=0.29; 95% CI: 0.15, 0.55; P<0.001*)1
- Median PFS in the OGSIVEO arm was not reached (95% CI: NR, NR) compared with 15.1 months (95% CI: 8.4, NR) in the placebo arm1,†,‡,§
- PFS events occurred in 12 patients (17%) in the OGSIVEO arm and 37 patients (51%) in the placebo arm1
- The Kaplan-Meier estimated median PFS for OGSIVEO could not be estimated due to the low number of events2
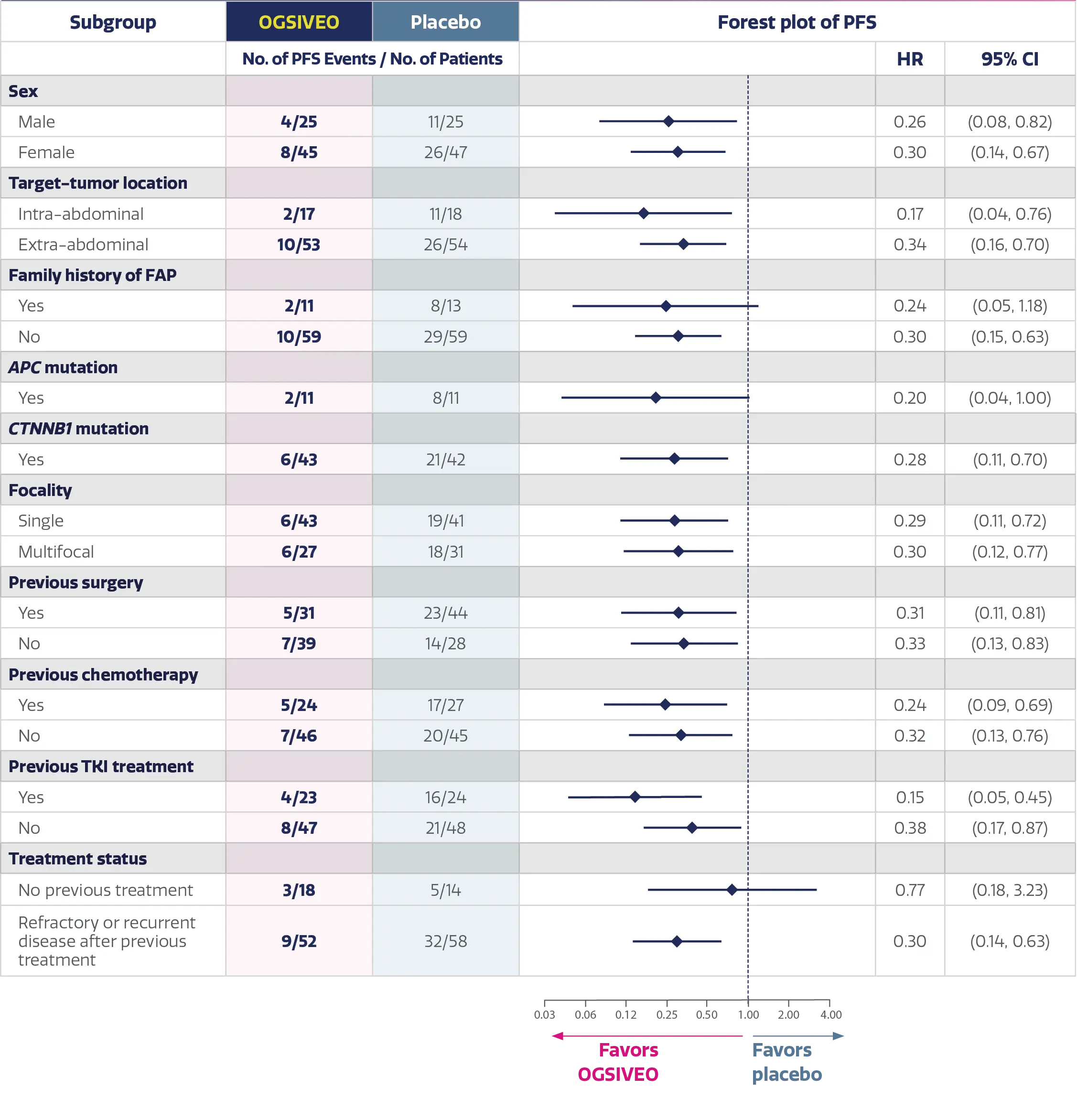
Subgroup analysis of the primary end point: Progression-free survival in
prespecified patient subgroups2
Additional analysis: PFS subgroups2,‖
Analysis Limitations
- DeFi was not powered to assess statistical differences between subgroups and this analysis should be considered descriptive only
- Therefore, the results require cautious interpretation and could represent chance findings
- These data are not included in the OGSIVEO Prescribing Information
P-value was from a one-sided stratified log-rank test with placebo as reference.1
Progression-free survival was defined as the time from randomization until the date of imaging-based or clinical progression or death. Progression-free survival was based on RECIST v1.1 as assessed by blinded independent central review or on clinical progression by the investigator (and confirmed by independent review). Clinical progression required worsening of symptoms resulting in a global deterioration of health status causing the permanent discontinuation from trial treatment and the initiation of emergent treatment (e.g., radiotherapy, surgery, or systemic therapy including chemotherapy or tyrosine kinase inhibitors) for desmoid tumors.1,2
Data cut-off as of April 7, 2022 for PFS.2
Obtained using Kaplan-Meier methodology.1
PFS was obtained using Kaplan-Meier methodology.2
APC, adenomatous polyposis coli; CI, confidence interval; CTNNB1, catenin beta 1; FAP, familial adenomatous polyposis; HR, hazard ratio; NR, not reached; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
OGSIVEO demonstrated a statistically significant improvement in objective response rate vs placebo1
Key Secondary Efficacy End Point:OGSIVEO treatment resulted in a 41% objective response rate with a 7% complete response rate1
Response definitions were based on RECIST criteria
- Objective response rate was defined as complete response or partial response according to RECIST v1.1. Assessed by blinded independent central review1,2
- Partial response was defined as a ≥30% decrease in the sum of the longest diameters of target tumors2
- Complete response was defined as disappearance of all target and non-target tumors2
Time to response (exploratory end point)
Median time to objective response was 5.6 months with OGSIVEO (range: 2.6 to 19.4 months) vs 11.1 months with placebo (range: 2.8 to 16.4 months).2,§
Analysis Limitations
- Time to objective response was an exploratory end point in the DeFi study
- This end point was not powered for statistical analysis and should be considered descriptive only
- Therefore, the results require cautious interpretation and could represent chance findings
- These data are not included in the OGSIVEO Prescribing Information
Objective response rates were consistent across prespecified subgroups2,3
Analysis Limitations
- DeFi was not powered to assess statistical differences between subgroups and these analyses should be considered descriptive only
- Therefore, the results require cautious interpretation and could represent chance findings
- These data are not included in the OGSIVEO Prescribing Information
97% of patients in the OGSIVEO arm who had a response were continuing to respond at the time of the analysis2
Examples of individual patient responses to OGSIVEO
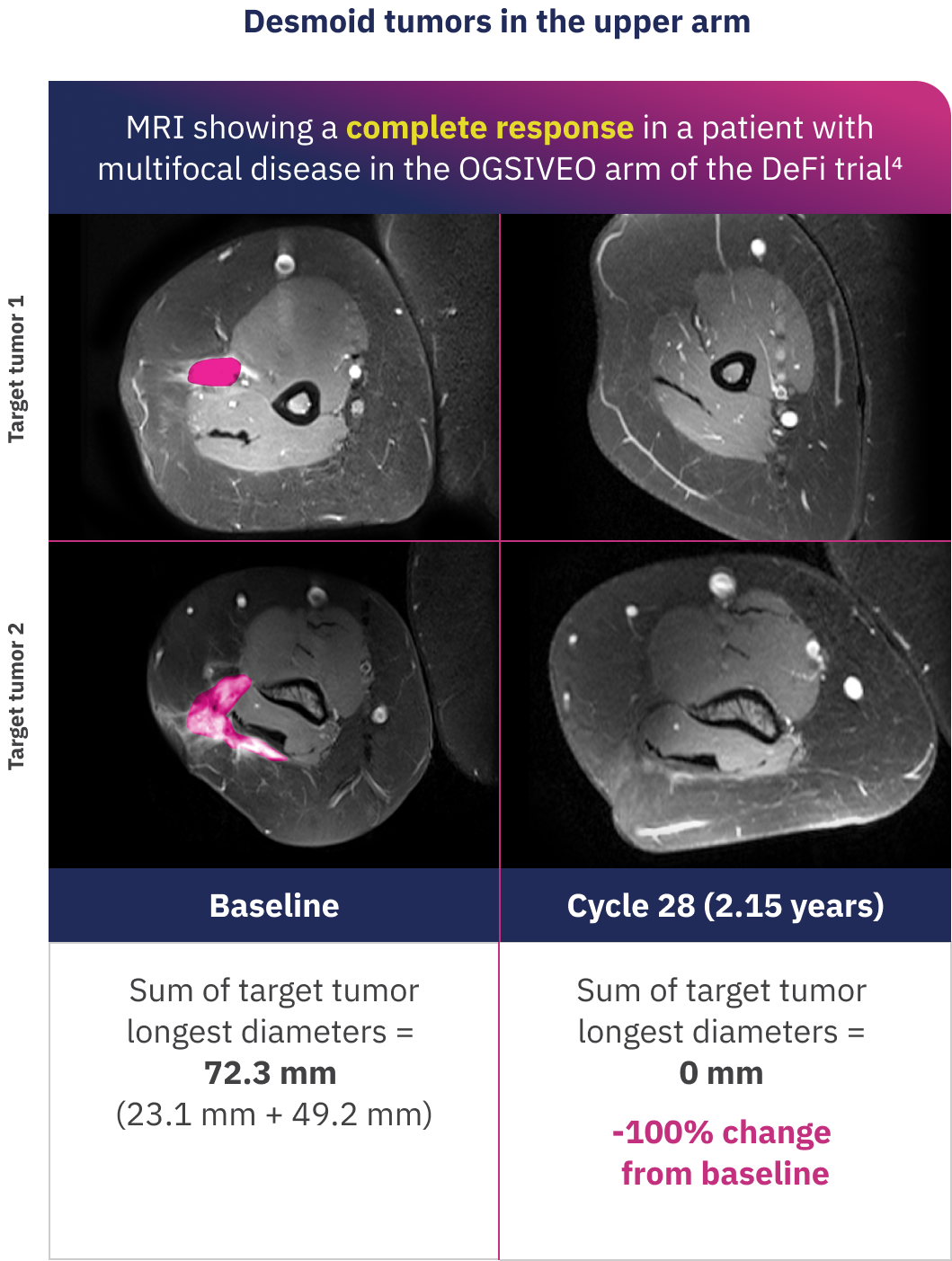
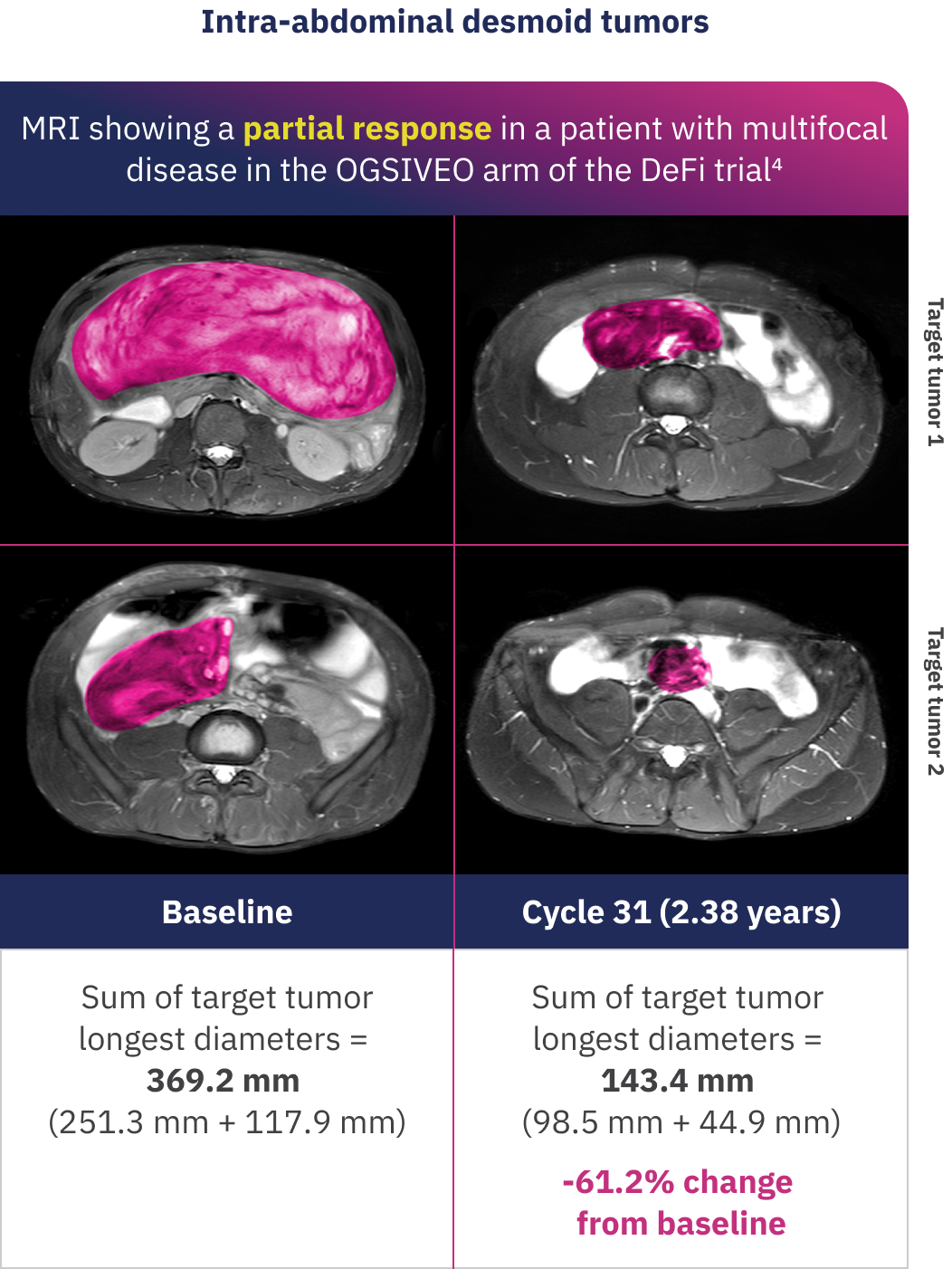
These MRI images show desmoid tumor responses for 2 patients who received OGSIVEO in the double-blind portion of the DeFi trial. Individual responses to OGSIVEO may vary. In the OGSIVEO arm of the DeFi trial, 7% of patients had a complete response and 34% of patients had a partial response.1
Images adapted with permission from Alcindor T, Kasper B, Gounder M, et al. Tumor volume and T2 hyperintensity changes from DeFi: a phase 3, randomized, controlled trial of nirogacestat in patients with desmoid tumors. Poster presentation at: ASCO Annual Meeting; June 2-6, 2023; Chicago, IL. False color added.
Tumor volume reductions with OGSIVEO (exploratory end point)
In DeFi, the median best percent change from baseline in tumor volume at any time post-treatment was -58.9% in the OGSIVEO arm (IQR: -84.7%, -8.9%) compared with +13.8% in the placebo arm (IQR: -26.1%, +58.6%).4,‖
Analysis Limitations
- Best percent change in tumor volume was an exploratory end point in the DeFi study. This end point was not powered for statistical analysis and should be considered descriptive only
- The use of MRI to assess changes in tumor volume represents a novel imaging technique
- Therefore, the results require cautious interpretation and could represent chance findings
- These data are not included in the OGSIVEO Prescribing Information
Best overall responses in patients who received OGSIVEO
Each bar in the chart below represents the best response of an individual patient in the OGSIVEO arm of the DeFi study.
Exploratory analysis: Best percent change in tumor size in the OGSIVEO arm2,*,¶,#
Dashed lines on graph indicate at least a 30% decrease or 20% increase in the sum of diameters of target lesions as indicated by the RECIST v1.1 response, taking as reference the baseline sum diameters.
The median best percent change in target-tumor size was -27.1% (range: -100 to 37) in the OGSIVEO arm compared with 2.3% (range: -100 to 47) in the placebo arm.2
Best overall responses in patients who received placebo
Each bar in the chart below represents the best response of an individual patient in the placebo arm of the DeFi study.
Exploratory analysis: Best percent change in tumor size in the placebo arm2,*,¶,#
Dashed lines on graph indicate at least a 30% decrease or 20% increase in the sum of diameters of target lesions as indicated by the RECIST v1.1 response, taking as reference the baseline sum diameters.
Analysis Limitations
- Best confirmed overall response was an exploratory end point in the DeFi study. This end point was not powered for statistical analysis and should be considered descriptive only
- Stable disease is not a component of ORR because it can reflect the natural history of the disease rather than a direct therapeutic effect
- Therefore, the results require cautious interpretation and could represent chance findings
- These data are not included in the OGSIVEO Prescribing Information
Desmoid tumors can have an unpredictable course and may exhibit spontaneous regression.5
Obtained using exact method based on binomial distribution.1
P-value was from a two-sided Cochran-Mantel-Haenszel test.1
Median time to objective response was calculated as time in months from first dose until date of the first documented response (CR or PR).2
Volumetric MRI (no contrast required) of each patient’s largest target tumor were evaluated at screening and every 6 cycles during the double-blind phase of the DeFi study. CT or MRI scans (investigator’s choice) were acquired to assess tumor changes. All scans for tumor volume were assessed by blinded independent central review.4
Waterfall plots show the best percent change at any time point from baseline in target-tumor size per patient according to RECIST v1.1 through blinded independent central review.2
Best confirmed overall response was the best response recorded from the start of the study treatment until disease progression/recurrence (taking as reference for progressive disease the smallest measurements recorded since the start of study treatment). The patient’s best response assignment depended on the achievement of both measurement and confirmation criteria. Values for best percent change were averaged between two independent reviewers unless a reader was selected for adjudication, in which case only the adjudicated value is presented.2
CR, complete response; CT, computed tomography; DeFi, Desmoid Fibromatosis; FAP, familial adenomatous polyposis; MRI, magnetic resonance imaging; ORR, objective response rate; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors.
OGSIVEO reduced patient-reported worst pain intensity1
PFS results were supported by change from baseline in patient-reported worst pain intensity favoring the OGSIVEO arm1
- Change from baseline at Cycle 10 in patient-reported worst pain intensity was a prespecified secondary end point in the DeFi study2
- Patient-reported outcome questionnaires for the BPI-SF were completed at baseline and the start of every treatment cycle2
- Assessment of pain at time points other than Cycle 10 was not prespecified in the DeFi study
- A positive change from baseline indicated worsening of patient-reported worst pain intensity2
BPI-SF Average Worst Pain Intensity Score2,*,†
Patient-reported worst pain intensity was assessed daily using item 3 of the BPI-SF, an 11-point numerical rating scale ranging from 0 (“no pain”) to 10 (“pain as bad as you can imagine”) and averaged over 7 days prior to each visit. Each cycle was 28 days. Cycle 10 was chosen as the time point for patient-relevant end point analysis to allow adequate time for a treatment response to be observed.1,2
Scheduled visits with at least 10 patients in each arm were included in the analysis.2
The PRO questionnaire was considered to be completed if the patients met the minimum requirements for scoring. Some patients discontinued and were not expected to complete the PRO questionnaire but did complete the minimum requirements for scoring.2
Analysis Limitations
- Definitive conclusions cannot be made about the BPI-SF results due to low questionnaire completion rate, asymmetric questionnaire completion between treatment arms, and the fact that analysis of results at time points other than Cycle 10 was not prespecified in the statistical analysis plan
- These data are not included in the OGSIVEO Prescribing Information
BPI-SF, Brief Pain Inventory-Short Form; C, cycle; CI, confidence interval; DeFi, Desmoid Fibromatosis; LS, least squares; PFS, progression-free survival; PRO, patient-reported outcome.
OGSIVEO safety profile
Warnings and Precautions1
Diarrhea
Diarrhea, sometimes severe, can occur in patients treated with OGSIVEO. In DeFi, diarrhea occurred in 84% of patients treated with OGSIVEO, and included Grade 3 events in 16% of patients. Median time to first diarrhea event for patients treated with OGSIVEO was 9 days (range: 2 to 434 days). Monitor patients and manage using antidiarrheal medications. Modify dose as recommended.
Ovarian Toxicity
Female reproductive function and fertility may be impaired in patients being treated with OGSIVEO. Impact on fertility may depend on factors including the duration of therapy and the state of gonadal function at the time of treatment. The long-term effects of OGSIVEO on fertility have not been established. Advise patients on the potential risks for ovarian toxicity before initiating treatment with OGSIVEO. Monitor patients for changes in menstrual cycle regularity or the development of symptoms of estrogen deficiency, including hot flashes, night sweats, and vaginal dryness.
Hepatotoxicity
ALT or AST elevations occurred in 30% and 33% of patients who received OGSIVEO in DeFi, respectively. Grade 3 ALT or AST elevations (>5 × ULN) occurred in 6% and 2.9% of patients, respectively. Monitor liver function tests regularly and modify dose as recommended.
Non-Melanoma Skin Cancers
New non-melanoma skin cancers can occur in patients treated with OGSIVEO. In DeFi, cutaneous squamous cell carcinoma and basal cell carcinoma occurred in 2.9% and 1.4% of patients, respectively. Perform dermatologic evaluations prior to initiation of OGSIVEO and routinely during treatment.
Electrolyte Abnormalities
Electrolyte abnormalities can occur in patients treated with OGSIVEO. In DeFi, these included decreased phosphate (65%) and decreased potassium (22%). Phosphate <2 mg/dL occurred in 20% of patients who received OGSIVEO. Grade 3 decreased potassium occurred in 1.4% of patients. Monitor phosphate and potassium levels regularly and supplement as necessary. Modify dose as recommended.
Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, OGSIVEO can cause fetal harm when administered to pregnant women. Oral administration of nirogacestat to pregnant rats during the period of organogenesis resulted in embryo-fetal toxicity and death at maternal exposures below the human exposure at the recommended dose of 150 mg twice daily. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DeFi, Desmoid Fibromatosis; ULN, upper limit of normal.
Adverse reactions in the DeFi trial
Most adverse events were Grade 1 or 2 and occurred within 1 month of starting OGSIVEO2
- 95% of adverse events were Grade 1 or 2 in the OGSIVEO arm of the DeFi trial2
- The first onset of adverse events in most patients treated with OGSIVEO occurred during Cycle 1 (within 28 days)2
- Serious adverse reactions occurred in 20% of patients who received OGSIVEO. Serious adverse reactions occurring in ≥2% of patients were ovarian toxicity (4%)1
- The most common adverse reactions (≥15% with a difference between arms of ≥5% compared to placebo) that occurred in patients receiving OGSIVEO were diarrhea, ovarian toxicity, rash, nausea, fatigue, stomatitis, headache, abdominal pain, cough, alopecia, upper respiratory tract infection, and dyspnea1
Adverse reactions (≥15%) in patients with desmoid tumor who received OGSIVEO with a difference between arms of ≥5% compared to placebo in DeFi1
Includes multiple related composite terms.1
Investigator assessment of ovarian toxicity included ovarian failure, premature menopause, amenorrhea, and menopause.1
The number of females of reproductive potential in each arm is used as the denominator (OGSIVEO N=36, placebo N=37).1
Negative value due to imputation rule at time of analysis.
- In the DeFi trial, the median duration of exposure for OGSIVEO was 20.6 months (range: 0.3 to 33.6 months)1
- Clinically relevant adverse reactions occurring in <15% of patients receiving OGSIVEO in DeFi included non-melanoma skin cancers, epistaxis, hidradenitis suppurativa, folliculitis, and influenza-like illness1
- In DeFi, the median time to first onset of skin rash was 22 days (range: 2 to 603 days) in patients who received OGSIVEO and the median duration was 37 days (range: 1 to 815 days). In the OGSIVEO arm of the DeFi trial, skin rash led to dose reduction in 9% of patients (4/47) and discontinuation in 2% (1/47) of patients3
- In DeFi, the median time to first onset of stomatitis (oral mucositis) in patients who received OGSIVEO was 9 days (range: 1 to 343 days) and the median duration was 33 days (range: -28 to 993 days).§ Eleven percent of patients with stomatitis on OGSIVEO (3/27) were dose reduced to 100 mg BID. No patients discontinued OGSIVEO due to stomatitis3
Proactive monitoring and management can help support patients receiving OGSIVEO
- Diarrhea: Median time to first diarrhea event for patients treated with OGSIVEO was 9 days (range: 2 to 434 days). Monitor patients and manage using antidiarrheal medications.‖ Modify dose as recommended. Advise patients to contact their HCP for sustained diarrhea that does not respond to supportive care1
- Ovarian Toxicity: The long-term effects of OGSIVEO on fertility have not been established. Advise patients on the potential risks for ovarian toxicity before initiating treatment with OGSIVEO. Monitor patients for changes in menstrual cycle regularity or the development of symptoms of estrogen deficiency, including hot flashes, night sweats, and vaginal dryness. Advise patients to tell their HCP if they experience these symptoms1
- Hepatotoxicity: Monitor liver function tests regularly and modify dose as recommended1
- Non-Melanoma Skin Cancers: Perform dermatologic evaluations prior to initiation of OGSIVEO and routinely during treatment. Advise patients to contact their HCP for any new or changing lesions on their skin1
- Electrolyte Abnormalities: Monitor phosphate and potassium levels regularly and supplement as necessary. Modify dose as recommended. Advise patients to contact their HCP if they experience muscle pain or weakness1
- Embryo-Fetal Toxicity: Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their HCP of a known or suspected pregnancy, and to stop taking OGSIVEO if they become pregnant. Advise females and males of reproductive potential to use effective contraception during treatment with OGSIVEO and for 1 week after the last dose1
Prior to use of any concomitant medication, please refer to Section 7 (Drug Interactions) of the OGSIVEO Prescribing Information.
BID, twice daily; DeFi, Desmoid Fibromatosis; HCP, healthcare professional.
Additional safety and tolerability data from the DeFi trial
Laboratory abnormalities (≥15%) reported in DeFi1
Dosage interruptions, dose reductions, and discontinuation due to an adverse reaction in patients who received OGSIVEO in the DeFi trial1
had dose interruptions
Adverse reactions which required dosage interruption in ≥2% of patients on OGSIVEO included diarrhea, rash, stomatitis, hypophosphatemia, fatigue, folliculitis, nausea, and ovarian toxicity
had dose reductions
Adverse reactions which required dose reductions in ≥2% of patients on OGSIVEO included diarrhea, rash, stomatitis, hypophosphatemia, folliculitis, hidradenitis, and ovarian toxicity
permanently discontinued
Adverse reactions which resulted in permanent discontinuation of OGSIVEO in ≥2% of patients were diarrhea, ovarian toxicity, increased ALT, and increased AST
The denominator used to calculate the rate was 69 for OGSIVEO and 72 for placebo based on the number of patients with a baseline value and at least one post-treatment value.1
CTCAE Version 5.0 does not include numeric thresholds for grading of hypophosphatemia; all grades represent patients with lab value < lower limit of normal (LLN).1
The denominator used to calculate the rate was 68 for OGSIVEO and 69 for placebo based on the number of patients with a baseline value and at least one post-treatment value.1
CTCAE Version 5.0 does not include numeric thresholds for grading of increased urine glucose.1
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; DeFi, Desmoid Fibromatosis.
Long-term post-hoc analysis of the DeFi study
- Patients from both the placebo and OGSIVEO treatment arms were eligible to enroll in the open-label extension phase and receive OGSIVEO 150 mg BID6
- A post-hoc analysis at the annual milestones of 1 (n=46), 2 (n=40), 3 (n=33), and 4 (n=15) years for patients who initially received OGSIVEO was conducted6,*
Objective response rate and tumor size reductions in a long-term analysis
Objective response rate was 45.7% and complete response rate
was 11.4% with OGSIVEO (up to 4-year exposure)3,6,†,‡

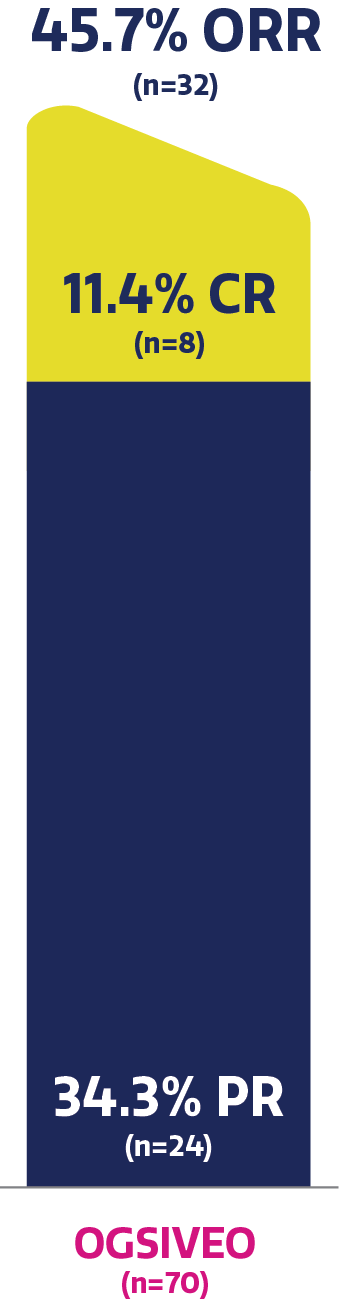
OSGIVEO exposure, median (range): 33.6 (0.3–60.0) months.6
Median best percent change in target-tumor size after each year of treatment with OGSIVEO6,†
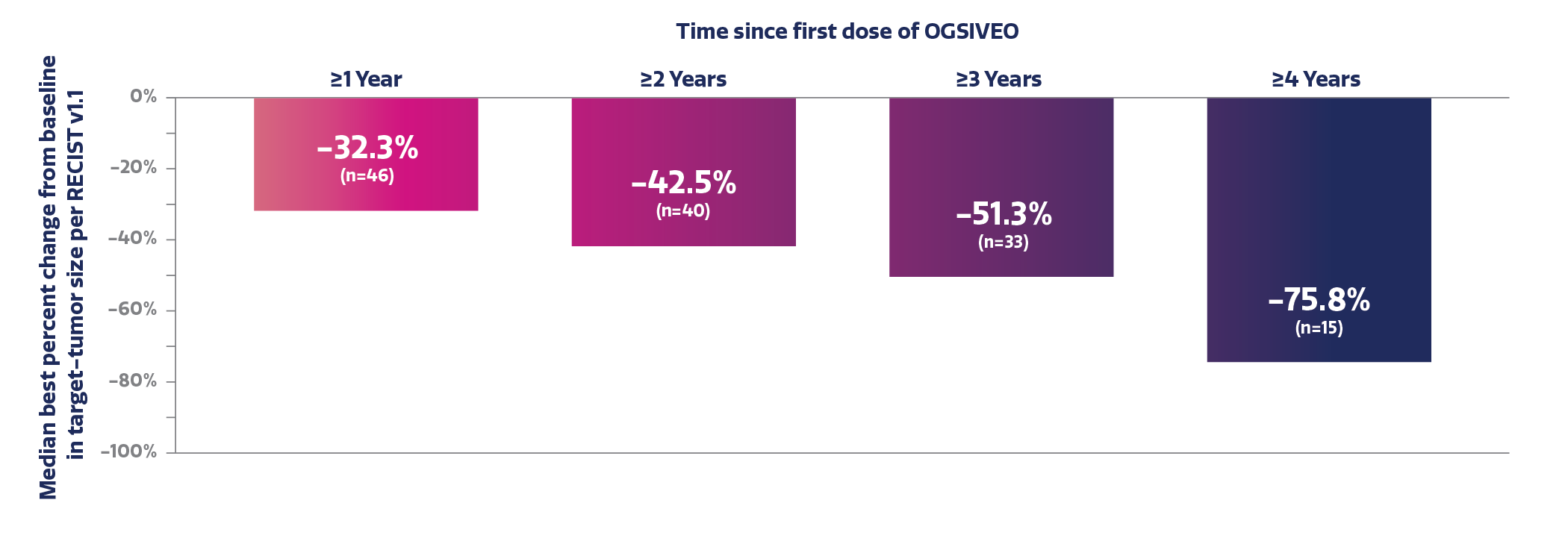
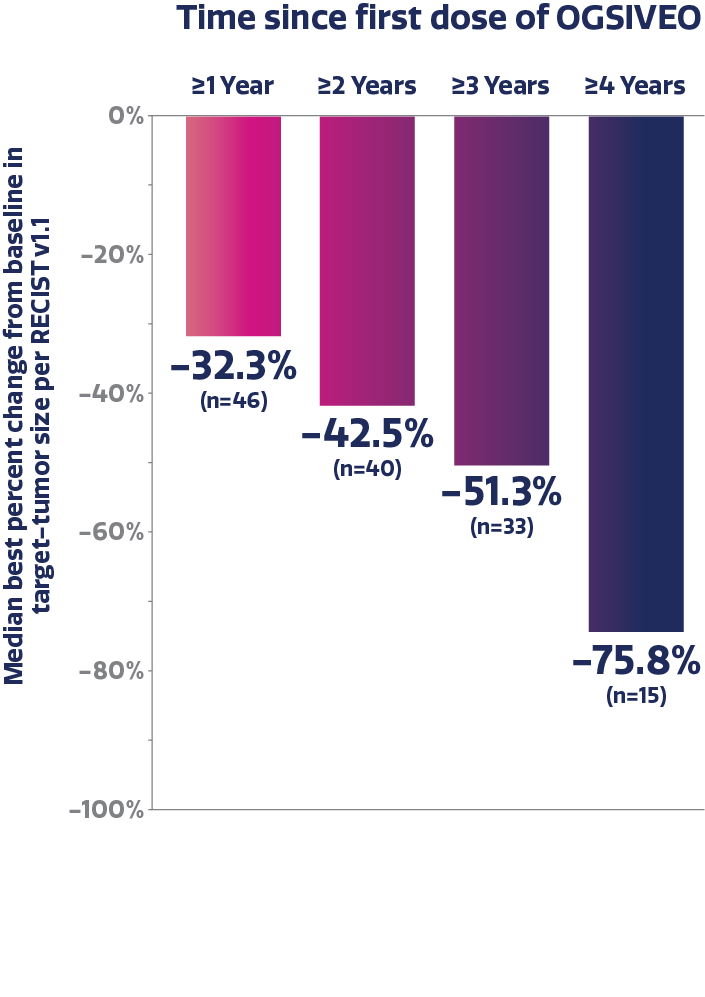
Patient-reported worst pain intensity in a long-term analysis
LS mean change from baseline in BPI-SF average worst pain intensity score ranged from -1.86 to -1.26 on a 0-10 scale.6,§
Adverse Events
After a median duration of 33.6 months (range: 0.3 to 60 months) of treatment with OGSIVEO:6
- The most frequently reported all-grade TEAEs were diarrhea, nausea, fatigue, hypophosphatemia, and headache
- Most adverse events were Grade 1 or 2, with the first onset occurring in the first year of treatment for most patients
- The overall incidence and severity of frequently reported adverse events decreased through years 2, 3, and 4 of treatment
Analysis Limitations
- Based on a post-hoc analysis in patients initially randomized to OGSIVEO in the DeFi study. In an open-label extension, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out. Extension data are as observed and should be considered descriptive in nature
- No conclusions of safety or efficacy should be made based on these results. These data are not included in the OGSIVEO Prescribing Information
Data cut-off date was August 13, 2024.6
Confirmed best response was defined as the best response obtained across all assessments considered per independent central review. PR and CR required confirmation by subsequent scans. The confirmed best response for patients while on OGSIVEO up to 4 years is presented. Objective response rate was defined as having a best response of complete response or partial response by RECIST v1.1. Obtained using exact method (Clopper-Pearson) based on binomial distribution.3,6
Objective response rate was assessed from randomization up to landmark years 1, 2, 3, and 4 of continuous OGSIVEO (inclusive of discontinuations prior to the landmark year).6
Based on the averaged results of 3-month intervals with DB and OLE data combined. For the subjects with DB phase last dose and OLE phase first dose gap >5 days, the gap was deducted for re-windowing.6
BID, twice daily; BPI-SF, Brief Pain Inventory-Short Form; CR, complete response; DB, double-blind; DeFi, Desmoid Fibromatosis; LS, least squares; OLE, open-label extension; ORR, objective response rate; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TEAE, treatment-emergent adverse event.


