What is OGSIVEO?
OGSIVEO is indicated for adult patients with progressing desmoid tumors who require systemic treatment. It is the first and only FDA-approved targeted gamma secretase inhibitor. OGSIVEO was evaluated in the DeFi study, a Phase 3 pivotal trial that included 142 adult patients with progressing desmoid tumors.1,2
Nirogacestat (OGSIVEO) is recommended as a treatment option by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)
NCCN Guidelines® for Soft Tissue Sarcoma recommend nirogacestat (OGSIVEO) as a Category 1 Preferred systemic therapy option for patients with desmoid tumors (aggressive fibromatosis).3
OGSIVEO is the #1 prescribed systemic therapy for adults with desmoid tumors4
DeFi, Desmoid Fibromatosis; FDA, US Food and Drug Administration; NCCN, National Comprehensive Cancer Network® (NCCN®).
DeFi Study Publication
OGSIVEO was evaluated in DeFi—the largest completed Phase 3 trial in adult patients with desmoid tumors. Review the results of this landmark study in The New England Journal of Medicine.
OGSIVEO offers convenient oral dosing1
The recommended dosage of OGSIVEO is 150 mg administered orally twice daily until disease progression or unacceptable toxicity

OGSIVEO may be taken with or without food

Instruct patients to swallow OGSIVEO tablets whole and not to break, crush, or chew prior to swallowing

If a patient vomits or misses a dose, instruct the patient to take the next dose at its scheduled time

Avoid concomitant use of OGSIVEO with grapefruit products, Seville oranges, and starfruit
OGSIVEO is supplied in 150 mg and 100 mg tablets
View OGSIVEO blister packaging and additional information
OGSIVEO is available in 150 mg and 100 mg tablets in blister packs
- Each blister pack contains a 7-day supply
- Four blister packs provide a 28-day supply
May help simplify tracking of AM/PM dosing



150 mg tablet
Tablets shown are not actual size.



100 mg tablet
Tablets shown are not actual size.
NDC numbers1
- 14-count blister pack (150 mg): 82448-150-14
- 14-count blister pack (100 mg): 82448-100-14
Storage and handling1
- Store at 20°C-25°C (68°F-77°F)
- Temperature excursions permitted between 15°C-30°C (59°F-86°F)
- See USP Controlled Room Temperature
Dosage modifications for OGSIVEO
The dose of OGSIVEO may be withheld then reduced to 100 mg twice daily for patients who experience certain adverse reactions.1
Dosage Modifications Table
Recommended dose modifications for adverse reactions1
(≥3 to 5 x ULN)
For other severe adverse reactions, life-threatening adverse reactions, or persistent intolerable Grade 2 adverse events, withhold drug until resolved to Grade ≤1 or baseline. Only restart at a dose of 100 mg twice daily after considering the potential benefit and likelihood of recurrence of the adverse reaction. Permanently discontinue OGSIVEO for recurrence of severe or life-threatening adverse reaction upon rechallenge at the reduced dose.1
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Drug Interactions With OGSIVEO
Exposure to OGSIVEO may be affected by other drugs.1
Effects of Other Drugs on OGSIVEO1
- Strong or moderate CYP3A inhibitors: OGSIVEO is a CYP3A substrate. Strong or moderate CYP3A inhibitors increase OGSIVEO exposure, which may increase the risk of OGSIVEO adverse reactions. Avoid concomitant use of OGSIVEO with strong or moderate CYP3A inhibitors, including grapefruit products, Seville oranges, and starfruit
- Strong or moderate CYP3A inducers: OGSIVEO is a CYP3A substrate. Strong or moderate CYP3A inducers decrease serum OGSIVEO exposure, which may reduce the effectiveness of OGSIVEO. Avoid concomitant use of OGSIVEO with strong or moderate CYP3A inducers
- Gastric acid reducing agents: OGSIVEO is poorly soluble at pH ≥6. Gastric acid reducing agents may decrease serum OGSIVEO exposure, which may reduce the effectiveness of OGSIVEO. Avoid concomitant use with proton pump inhibitors and H2 blockers. If concomitant use cannot be avoided, OGSIVEO can be staggered with antacids (e.g., administer OGSIVEO 2 hours before or 2 hours after antacid use)*,†
- For additional information about potential drug interactions with OGSIVEO, see Table 4 (Section 7.1) and Table 5 (Section 7.2) of the full Prescribing Information
Gastric acid reducing agents include PPIs (e.g., omeprazole, lansoprazole, esomeprazole), H2 blockers (e.g., cimetidine, famotidine, nizatidine), and antacids (e.g., Mylanta®, Rolaids®, Tums®). In accordance with the guidance in the OGSIVEO Prescribing Information, antacids should be administered either 2 hours before or 2 hours after OGSIVEO.
Prior to use of any concomitant medication, please refer to Section 7 (Drug Interactions) of the OGSIVEO Prescribing Information.
PPIs, proton pump inhibitors.
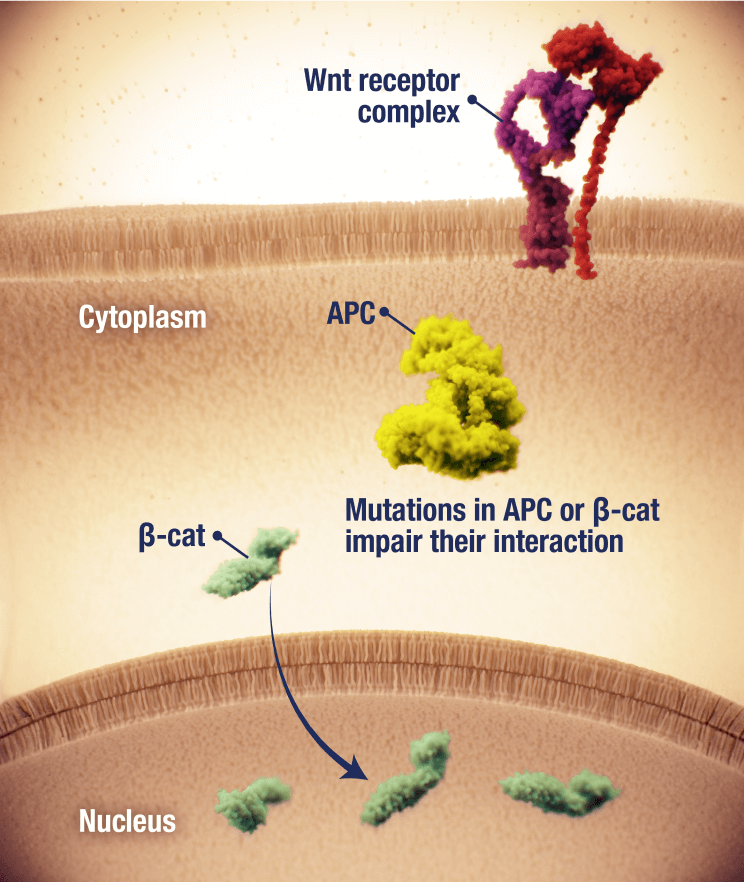
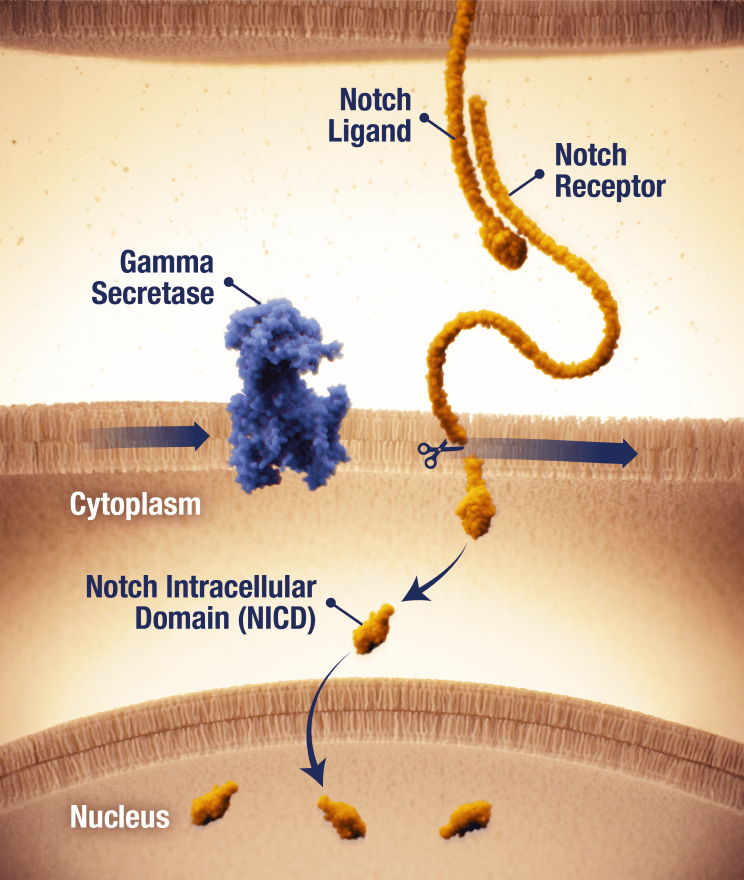
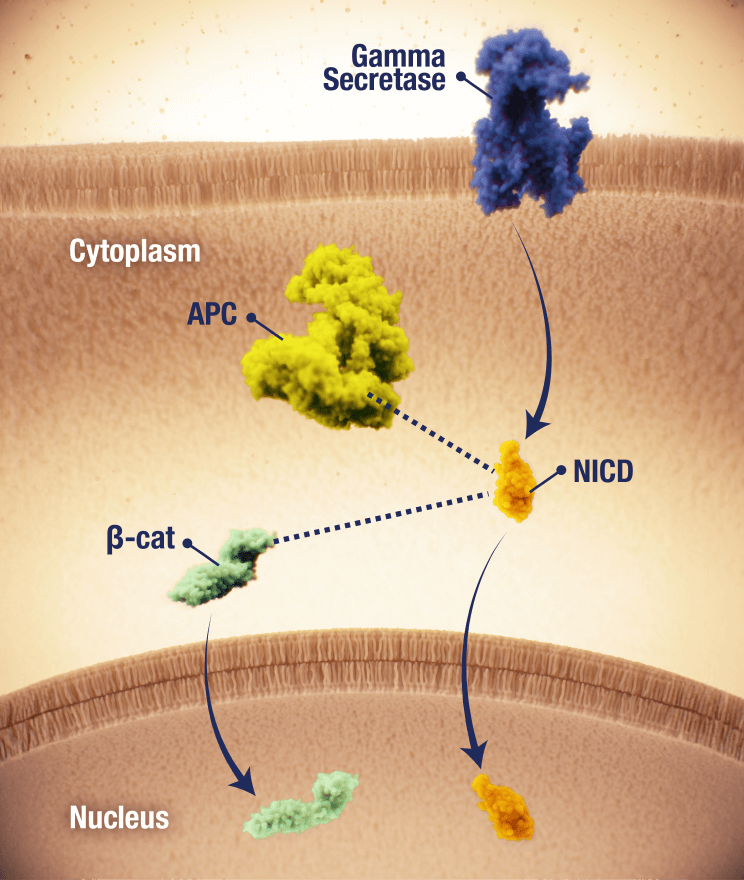
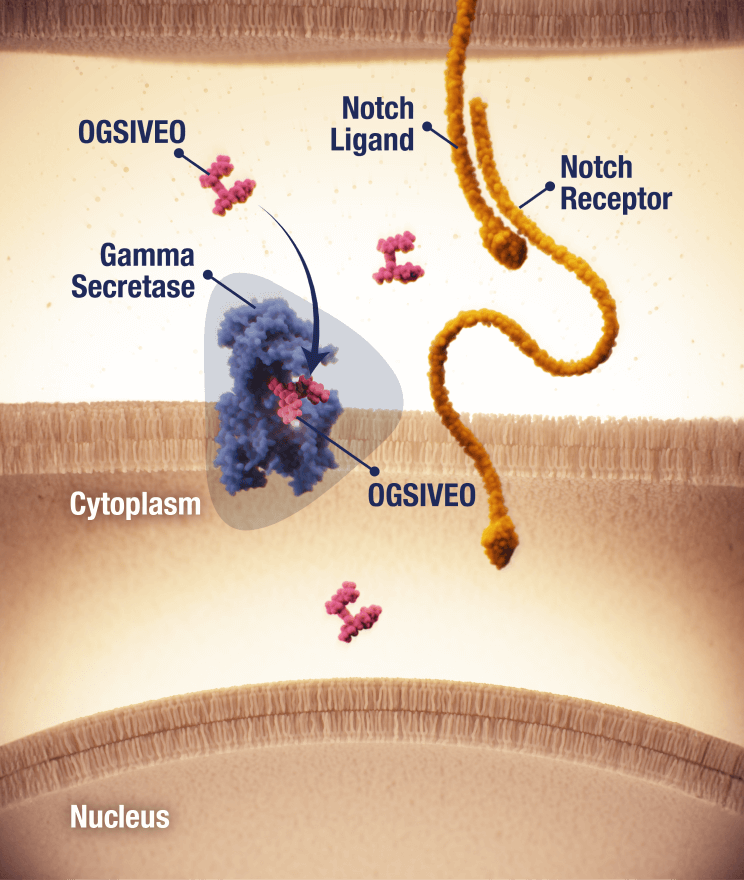
OGSIVEO is the first and only FDA-approved targeted gamma secretase inhibitor
Proposed mechanism of action based on in vitro models
APC, adenomatous polyposis coli; CTNNB1, catenin beta 1.