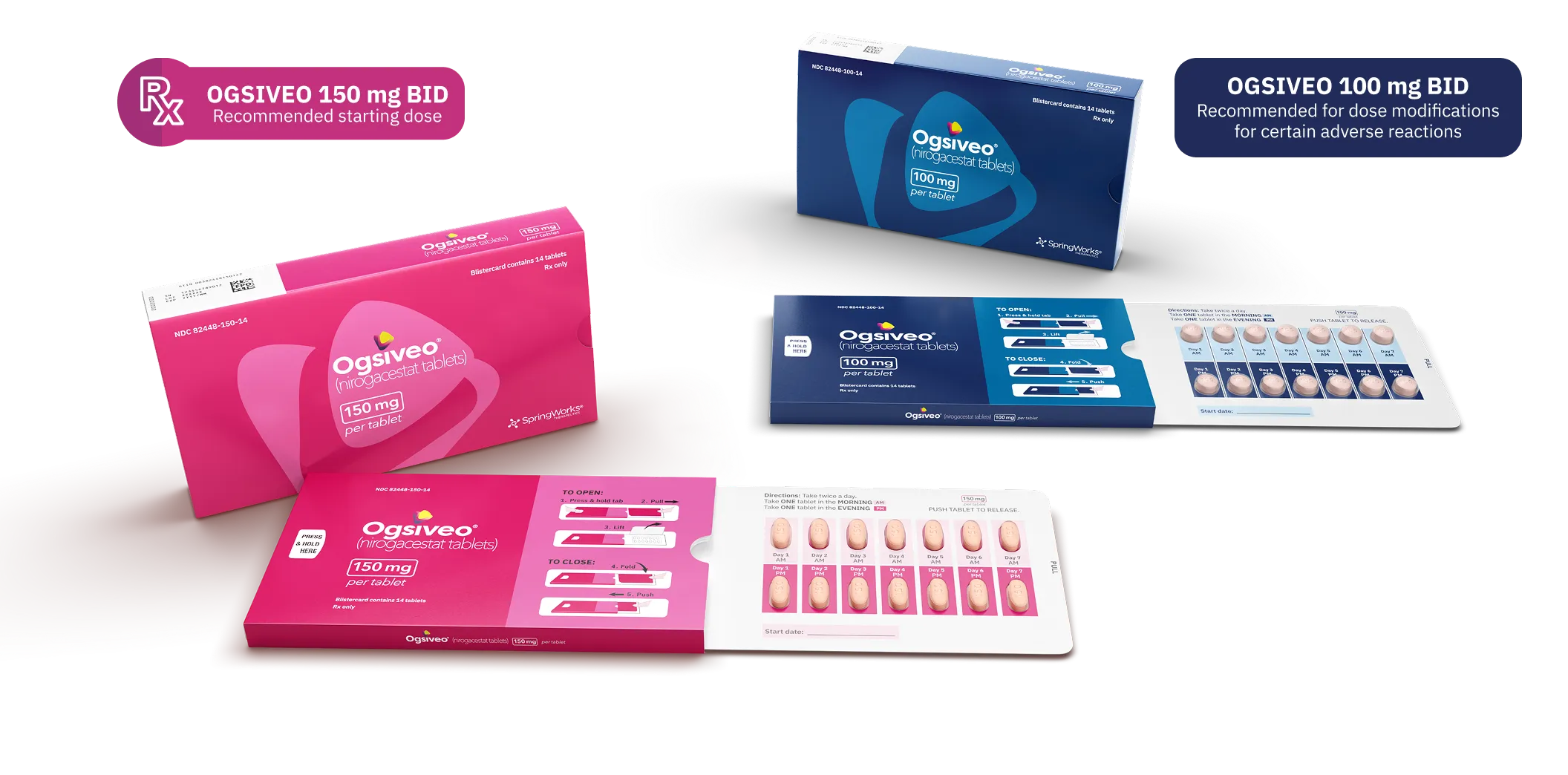
OGSIVEO offers convenient oral dosing1
The recommended dosage of OGSIVEO is 150 mg administered orally twice daily until disease progression or unacceptable toxicity

OGSIVEO may be taken with or without food

Instruct patients to swallow OGSIVEO tablets whole and not to break, crush, or chew prior to swallowing

If a patient vomits or misses a dose, instruct the patient to take the next dose at its scheduled time

Avoid concomitant use of OGSIVEO with grapefruit products, Seville oranges, and starfruit
OGSIVEO is available in 150 mg and 100 mg tablets in blister packs
- Each blister pack contains a 7-day supply
- Four blister packs provide a 28-day supply
May help simplify tracking of AM/PM dosing
Tablets shown are not actual size. Packages shown are for illustration only.
NDC numbers1
- 14-count blister pack (150 mg): 82448-150-14
- 14-count blister pack (100 mg): 82448-100-14
Storage and handling1
- Store at 20°C-25°C (68°F-77°F)
- Temperature excursions permitted between 15°C-30°C (59°F-86°F)
- See USP Controlled Room Temperature
Recommended Dosing
The recommended starting dose of OGSIVEO is 150 mg BID until disease progression or unacceptable toxicity.1
Dose modifications
For patients who experience certain adverse reactions, the dose of OGSIVEO may be withheld then reduced to 100 mg BID.1
BID, twice daily.

Packages shown are for illustration only.
Drug Interactions With OGSIVEO
Exposure to OGSIVEO may be affected by other drugs1
- Strong or moderate CYP3A Inhibitors: OGSIVEO is a CYP3A substrate. Strong or moderate CYP3A inhibitors increase OGSIVEO exposure, which may increase the risk of OGSIVEO adverse reactions. Avoid concomitant use of OGSIVEO with strong or moderate CYP3A inhibitors, including grapefruit products, Seville oranges, and starfruit
- Strong or moderate CYP3A Inducers: OGSIVEO is a CYP3A substrate. Strong or moderate CYP3A inducers decrease serum OGSIVEO exposure, which may reduce the effectiveness of OGSIVEO. Avoid concomitant use of OGSIVEO with strong or moderate CYP3A inducers
- Gastric acid reducing agents: OGSIVEO is poorly soluble at pH ≥6. Gastric acid reducing agents may decrease serum OGSIVEO exposure, which may reduce the effectiveness of OGSIVEO. Avoid concomitant use with proton pump inhibitors and H2 blockers. If concomitant use cannot be avoided, OGSIVEO can be staggered with antacids (e.g., administer OGSIVEO 2 hours before or 2 hours after antacid use)*,†
- For additional information about potential drug interactions with OGSIVEO, see Table 4 (Section 7.1) and Table 5 (Section 7.2) of the full Prescribing Information
Consult the full Prescribing Information prior to and during treatment for important drug interactions.
Gastric acid reducing agents include PPIs (e.g., omeprazole, lansoprazole, esomeprazole), H2 blockers (e.g., cimetidine, famotidine, nizatidine), and antacids (e.g., Mylanta®, Rolaids®, Tums®). In accordance with the guidance in the OGSIVEO Prescribing Information, antacids should be administered either 2 hours before or 2 hours after OGSIVEO.
Prior to use of any concomitant medication, please refer to Section 7 (Drug Interactions) of the OGSIVEO Prescribing Information.
PPIs, proton pump inhibitors.
How to Order and Prescribe
OGSIVEO is available through a limited Specialty Pharmacy Network. OGSIVEO is also available for eligible medically integrated dispensing pharmacies by ordering directly through one of our Specialty Distributor partners.
How to Order/Rx Guide
Discover how to prescribe OGSIVEO for your adult patients with progressing desmoid tumors who require systemic treatment.

You can prescribe OGSIVEO using one of the following options:
SpringWorks CareConnections – Complete the enrollment form, including the Prescription for OGSIVEO, choose a fulfillment option from our in-network Specialty Pharmacy partners, and OGSIVEO will be shipped directly to the patient.
Prescribe directly through a Specialty Pharmacy network or medically integrated dispensing (MID) pharmacy – Eligible MID pharmacy practices may purchase OGSIVEO direct through our Specialty Distributors.‡
May not be available for all practices or organizations.
100 mg tablets – 10288282
100 mg tablets – 5917596
100 mg tablets – 5017348
100 mg tablets – 2932218
Share the Patient Brochure
This brochure can help your patients learn about OGSIVEO, including efficacy and safety information, dosing instructions, and more.

Resources & Support for Patients
Encourage patients to take advantage of resources available to them:
References
- OGSIVEO. Prescribing Information. SpringWorks Therapeutics, Inc.
- Gounder M, Ratan R, Alcindor T, et al. Nirogacestat, a gamma-secretase inhibitor for desmoid tumors. N Engl J Med. 2023;388(10):898-912.
- Alcindor T, Kasper B, Gounder M, et al. Tumor volume and T2 hyperintensity changes from DeFi: a phase 3, randomized, controlled trial of nirogacestat in patients with desmoid tumors. Poster presentation at: ASCO Annual Meeting; June 2-6, 2023; Chicago, IL


