
What is OGSIVEO?
OGSIVEO is the #1 prescribed systemic therapy for adults with desmoid tumors.2
Help treat your desmoid tumor with the first and only FDA-approved treatment that can help get progressing desmoid tumors under control for adults who require a medicine by mouth or injection (systemic therapy). It is not known if OGSIVEO is safe and effective in children.1
Actor portrayal. Individual results may vary.
Guideline Recommendation
The NCCN* Guidelines for Patients® recommend nirogacestat (OGSIVEO) as a treatment option for desmoid tumors that are growing and/or causing symptoms.3
The National Comprehensive Cancer Network® (NCCN®) is a not-for-profit alliance of leading cancer centers devoted to patient care, research, and education.
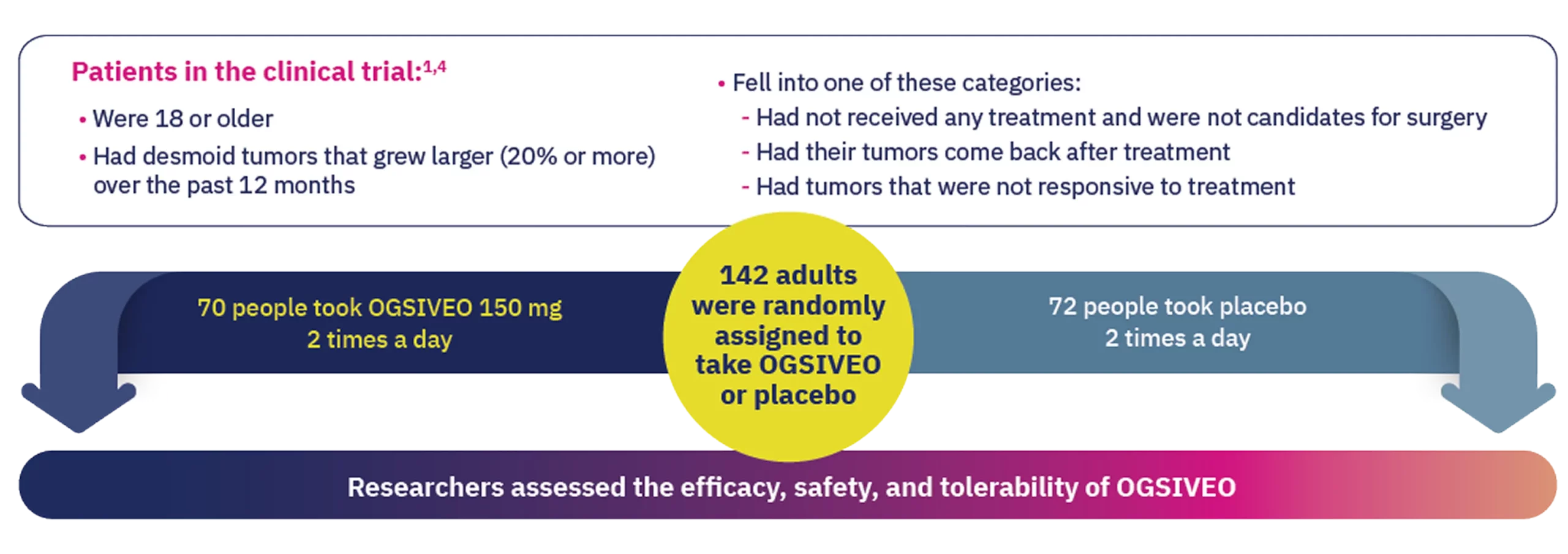
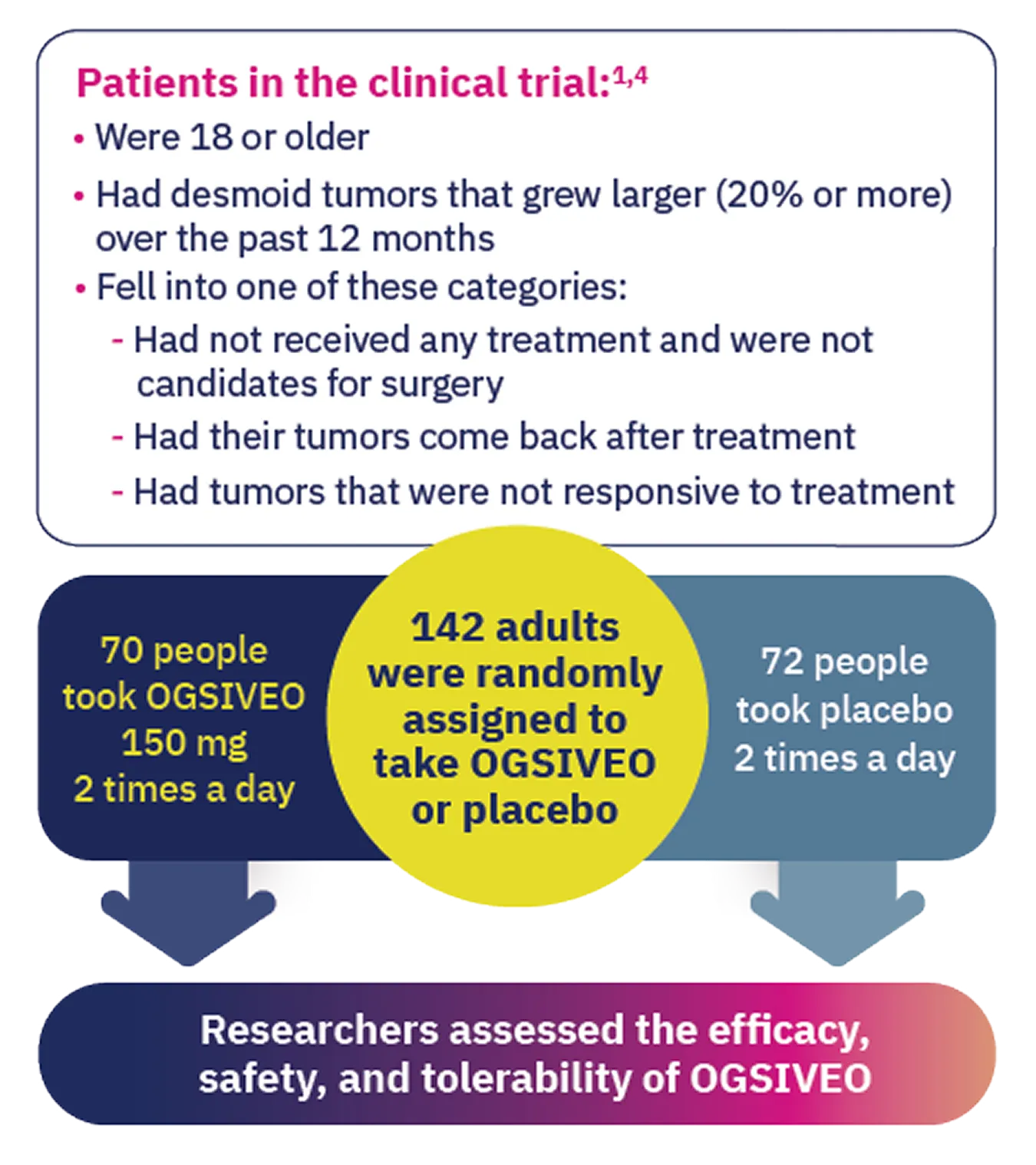
OGSIVEO was studied in the largest completed clinical trial of an FDA-approved treatment for adult patients with desmoid tumors.1,4
The trial compared OGSIVEO to placebo (a pill with no active medicine) in 142 adults. 70 people received OGSIVEO, and 72 people received placebo.
OGSIVEO is shown to be effective and safe—with impactful clinical trial results1,†
OGSIVEO lowered the chance of tumor progression or death
OGSIVEO shrank tumors for some people and, in certain cases, tumors disappeared
Patients reported reduced pain with OGSIVEO
In the study, progression could mean one of two things: 1) The tumor was growing bigger by 20% or more, or 2) The person felt new or worsening symptoms that caused them to stop treatment and leave the study, and start a different type of treatment for the desmoid tumor(s).1,4
Learn more about OGSIVEO
Download a patient brochure for information on OGSIVEO including its possible benefits and side effects.
OGSIVEO provided effective desmoid tumor control
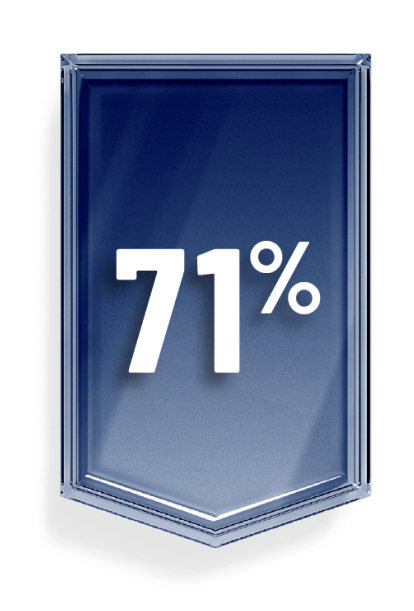
In the clinical trial, OGSIVEO reduced the chance of a participant’s disease getting worse during the study by 71% compared with a placebo.
This is known as progression-free survival.1,4
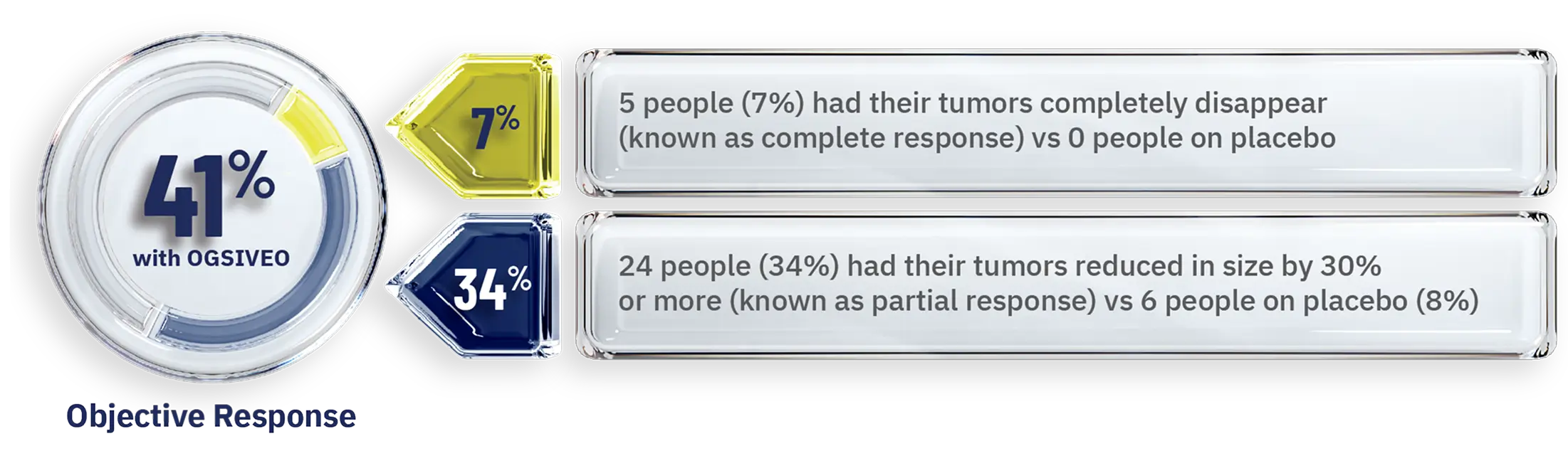
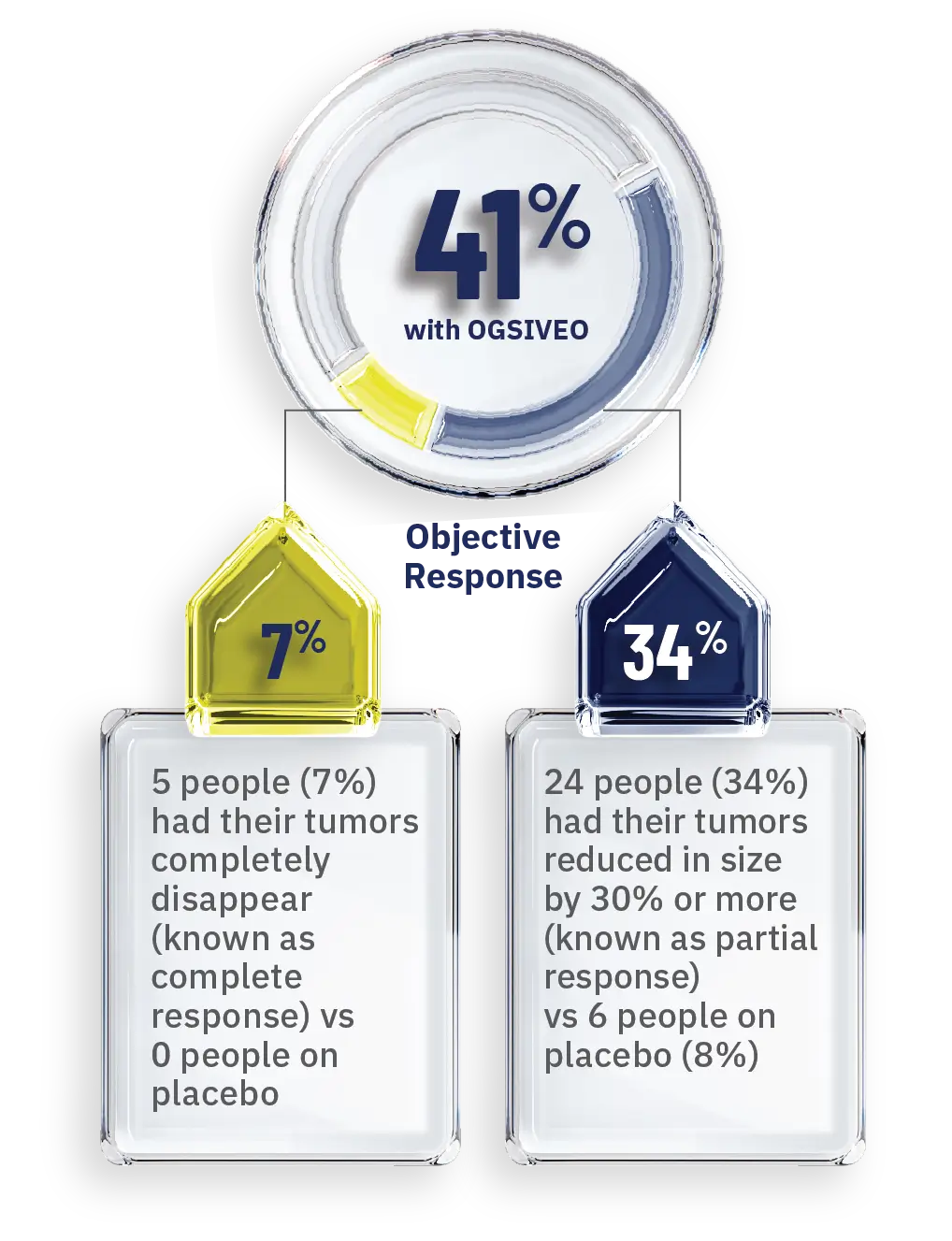
OGSIVEO also helped shrink desmoid tumors or caused them to completely disappear in some people1
Percentage of people whose tumors responded—as confirmed by MRI or CT scans
- When a tumor shrinks by 30% or more, this is known as an objective response.1,4
In patients who responded to OGSIVEO, tumors began to shrink between 2.6 and 19.4 months after starting therapy. The median (middle) amount of time it took for tumors to begin shrinking was 5.6 months, compared to 11.1 months (2.8 to 16.4 months) for patients taking placebo.4
Limitations: This was an exploratory analysis, meaning it was not specifically designed to find differences between OGSIVEO and placebo. Therefore, these results may be due to chance and should be interpreted carefully. Individual results may vary from the clinical trial experience.
Patients reported reduced pain—one of the most common symptoms of desmoid tumors—with OGSIVEO1,4

People filled out questionnaires at different points during the trial
- More than 50% of patients entering the clinical trial had pain. Over the course of the trial, people continued to report their pain
- People taking OGSIVEO reported reductions in worst pain compared with those taking placebo
Limitations: Meaningful changes in pain levels were difficult to estimate in the clinical trial because not all patients completed the pain questionnaires at each timepoint, and because the number of patients completing questionnaires differed between OGSIVEO and placebo treatment groups. Definitive conclusions cannot be made about the impact of OGSIVEO on pain.

After four years, my oncologist recommended OGSIVEO. After six months, the scans showed that OGSIVEO had helped get my desmoid tumor size under control.
I’ve noticed improvements in pain since I started my treatment. I do have prescription pain medication for occasional flare-ups, but thankfully it’s needed much less often.
Watch Christina’s full story and hear other patient voices.
Individual results may vary.

Create a Customized Doctor Discussion Guide
Create your own customized Doctor Discussion Guide to prepare for your next appointment. This guide can help you start the conversation with your care team.
The safety of OGSIVEO was evaluated in the clinical trial1
OGSIVEO can cause serious side effects, including:1
- Diarrhea
- Ovarian problems
- Liver problems
- New non-melanoma skin cancers
- Electrolyte (salt) problems
OGSIVEO can affect fertility in females and males, which may affect your ability to have a child.
Talk to your healthcare provider if this is a concern for you.1
These are not all of the possible side effects of OGSIVEO. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.1
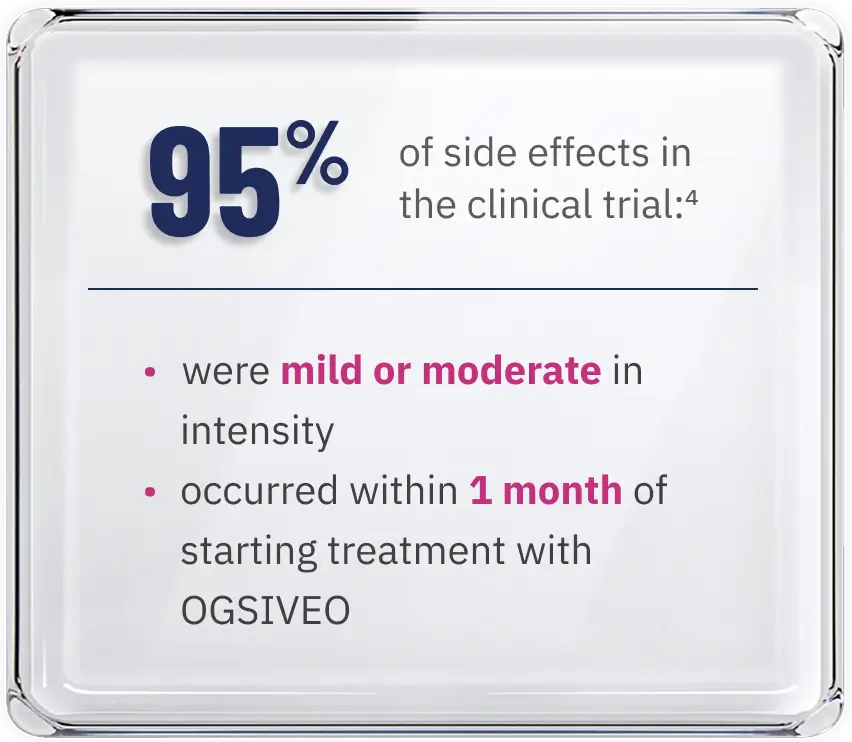
of side effects in the clinical trial:4
- were mild or moderate in intensity
- occurred within 1 month of starting treatment with OGSIVEO
If you think you may be experiencing any side effects, call your healthcare provider. Your healthcare provider may recommend medications or other approaches to help you manage side effects. Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with OGSIVEO.
Learn more about possible side effects and what you may expect from OGSIVEO.
OGSIVEO is a systemic therapy
Systemic therapies work throughout the entire body1,5—unlike surgery which is a local therapy, meaning it is typically focused on a single place in the body.6
OGSIVEO is not a chemotherapy. It is thought to work by interfering with gamma secretase—a protein that helps some type of cells grow, including desmoid tumor cells. OGSIVEO may keep desmoid tumor cells from growing.2,7 The precise mechanism of how OGSIVEO works is not known.
Ready to discuss OGSIVEO with your doctor?
Frequently asked questions about OGSIVEO
What is OGSIVEO?
OGSIVEO is a prescription medication used to treat adults with progressing desmoid tumors who require a medicine by mouth or injection (systemic therapy). It is not known if OGSIVEO is safe and effective in children.1
OGSIVEO is a systemic therapy, meaning it works throughout the entire body1,5—unlike surgery which is a local therapy, meaning it is typically focused on a single place in the body.6
Is OGSIVEO chemotherapy?
OGSIVEO is not a chemotherapy. It is a systemic therapy, meaning it works throughout the entire body1,5—unlike surgery which is a local therapy, meaning it is typically focused on a single place in the body.6
OGSIVEO is thought to work by interfering with gamma secretase—a protein that helps some types of cells grow, including desmoid tumor cells. OGSIVEO may keep desmoid tumor cells from growing.2,7 The precise mechanism of how OGSIVEO works is not known.
Is OGSIVEO injected?
No, OGSIVEO is an oral medicine. The tablets are taken by mouth twice a day, with or without food.1
How is OGSIVEO typically taken?
OGSIVEO is convenient to take at home. The recommended dosage of OGSIVEO is 150 mg taken 2 times
a day.1
OGSIVEO blister packs may help you track your dose more easily with clear AM/PM dosing by day of the week. Each blister pack contains a 7-day supply, and four blister packs provide a 28-day supply.
What should I tell my doctor before taking OGSIVEO?
Tell your healthcare provider about all of your medical conditions, including if you have liver problems, are pregnant or plan to become pregnant, and if you are breastfeeding or plan to breastfeed. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. You should avoid taking proton pump inhibitors (PPIs) and H2 blockers during treatment with OGSIVEO. Ask your healthcare provider if you are not sure if you take one of these medicines.1
How long will I take OGSIVEO?
Talk to your doctors about how long you might take OGSIVEO. In general, your doctor might recommend that you keep taking OGSIVEO unless your tumor grows (progresses), your symptoms worsen, or you have troublesome side effects that cannot be managed, as determined with your doctor. Follow your doctor’s recommendations for how to take OGSIVEO. During the clinical trial, people took OGSIVEO for a time period ranging from less than 1 month to over 2.5 years.1
Is OGSIVEO for anyone with a desmoid tumor?
No. OGSIVEO is for adults with progressing desmoid tumors who require systemic therapy. It is not known if OGSIVEO is safe and effective in children.1 Talk to your doctor to find out if OGSIVEO might be an option for you.

Sign up for information & resources
Sign up for information, tips, and resources to help you talk to your doctor as you navigate your treatment journey.

References
- OGSIVEO. Prescribing Information. SpringWorks Therapeutics, Inc.
- Data on file. SpringWorks Therapeutics, Inc. June 2025.
- Referenced with permission from the NCCN Guidelines for Patients® for Soft Tissue Sarcoma, 2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed August 7, 2025. To view the most recent and complete version of the NCCN Guidelines for Patients, go online to NCCN.org/patientguidelines. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application, and disclaims any responsibility for their application or use in any way.
- Gounder M, Ratan R, Alcindor T, et al. Nirogacestat, a gamma-secretase inhibitor for desmoid tumors. N Engl J Med. 2023;388(10):898-912.

